Your medical device benefits from cleanroom manufacturing and assembly
The growing need for quality manufacturing processes drives the need for more medical devices to be produced in a clean and regulated environment. You need a reliable manufacturing partner that adheres to strict industry regulations. Manufacturers with certified cleanrooms and white rooms meet those demands.
What is a Cleanroom?
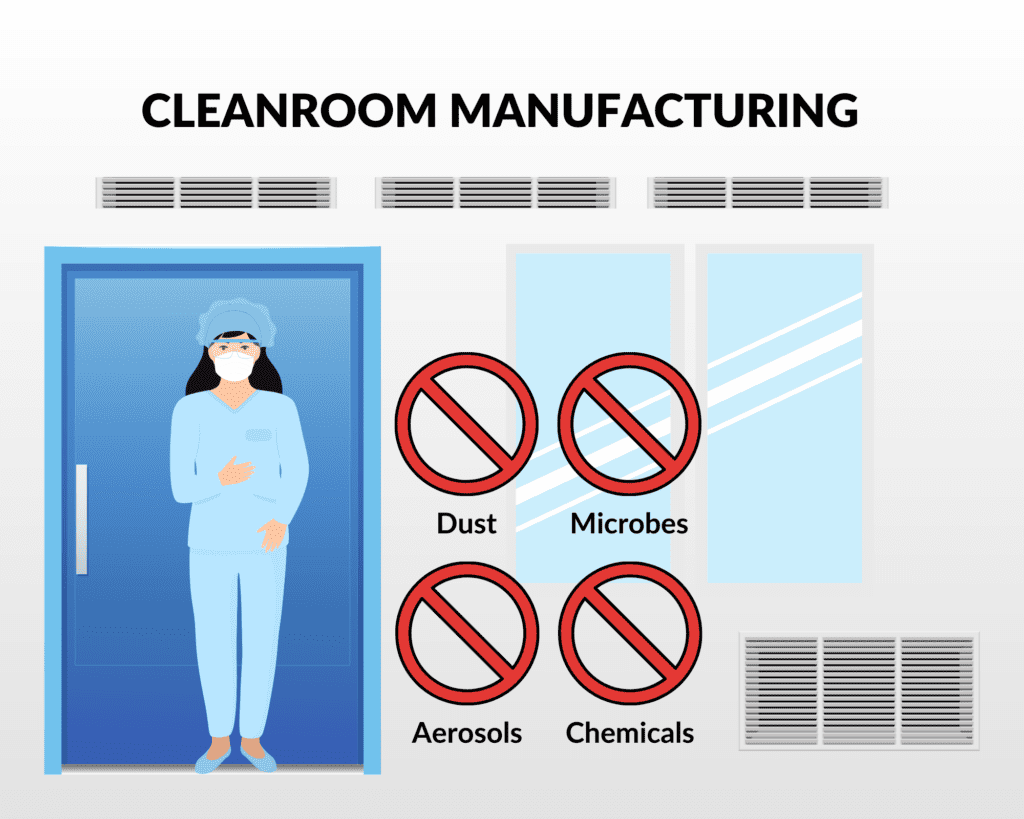
As medical device manufacturers, it is essential to have a space that is constantly working to remove foreign particles and contaminants from the air to ensure products are manufactured and assembled with the utmost quality.
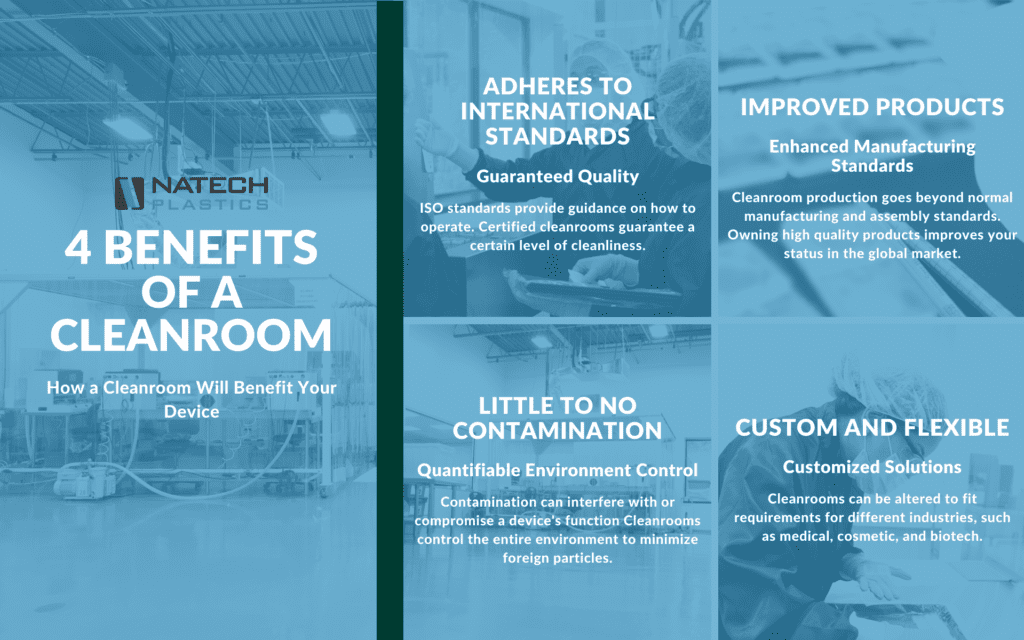
Cleanrooms aim to remove and limit airborne particles, such as dust, microbes, and other aerosols. Typical manufacturing spaces aren’t held to the same standards of cleanliness as cleanrooms. Cleanrooms have the advantage of environment control and enhanced manufacturing standards, which guarantee higher quality for your product.
Cleanrooms have maximum concentration limits for particles of certain sizes. For example, an ISO Class 8 cleanroom can allow a maximum of 100,000 particles (≥0.5 um) per cubic foot of air. You want to make sure to find the ISO Cleanroom Class that aligns with your part and budgetary requirements.
Is a Cleanroom Right for My Project?
Customers who typically need cleanrooms are producing products such as:
- Medical devices
- Pharmaceuticals
- Biotechnology
1. Medical devices
Typical ISO Classes: Class 7 to 8
Cleanroom environments are common for manufacturing and assembling medical devices. The cleanroom standards ensure the part surface does not have particulate contamination. To guarantee this, cleanroom staff are required to wear gowns and coverings like hairnets, gloves and masks.
2. Pharmaceuticals
Typical ISO Classes: Class 5 to 8
Similar to medical device cleanrooms, many pharmaceutical industry cleanrooms require ISO Class standards. If you’re concerned about particulate contamination, bacterial growth or cross contamination, cleanrooms can control factors like pressure, temperature, and humidity to prevent these issues.
3. Biotechnology
Typical ISO Classes: Class 5 to 8
If your biotechnology product involves handling fluids or other organic matter, cleanrooms keep out particulates that could contaminate test results or affect samples.
Many customers that require packaging and assembling of medical devices will need a cleanroom with some sort of specific ISO classification.
Conversely, some devices might not need a cleanroom, but still require a controlled environment that meets customer requirements for particle contamination.
It is important to ask if the standards set by a cleanroom facility are necessary for your product. Cleanroom facilities might be essential for certain medical device, pharmaceutical, or biotech components. Contact one of our engineers to understand if you need a cleanroom for your product.
Download your free cleanroom checklist to know if a cleanroom is right for your application.
How does Natech Ensure Quality and Cleanliness of Our Cleanroom?
Foreign particulates can be brought into the space via anything transported into the room, including people. Natech keeps our ISO Class 8 Cleanroom up to standard with proper gowning and appropriate practices to keep out particulates.
Team members are trained and guided by our quality department to make sure they develop and implement efficient processes and ensure requirements, specifications, and deadlines are met.

Anyone who enters our cleanroom must perform proper preliminary practices. To enter, operators cannot wear any perfume, heavily scented products, or heavy makeup. Any outerwear like jackets, sweatshirts, snow boots, or gloves must be removed, to ensure foreign particulates don’t get in. This also involves properly gowning with approved hair covers, gowns, face masks, wearing shoe covers and using a sticky mat to remove extra particles, using safety glasses, and gloves before entering the cleanroom.
Natech adheres to the ISO Class 8 Cleanroom standards. ISO standards provide guidance on how cleanrooms can operate and if it can remain certified. Because of this, our cleanroom guarantees a specific level of cleanliness in accordance with these standards and the probability of quality issues due to dirt or handling are much lower.
For example, Natech has used our cleanroom space to manufacture and assemble IVD (in vitro diagnostic) devices. A cleanroom was necessary because particulate could affect the end use of the product.
What Does Natech Offer?
Natech offers quality, in-house production services that come from decades of experience working in medical device manufacturing.
Our cleanroom can be used for the manufacture or assembly of many medical, pharmaceutical and biotech devices. Our cleanroom controls temperature, humidity, and air filtration to qualify it to be an ISO Class 8 Cleanroom.
Our customers produce life-critical devices and have confidence in the quality of the designs and manufactured product they receive from Natech. If your product requires cleanroom manufacturing, Natech provides value-added services that will address your manufacturing, assembling, and packaging needs.
Schedule time with an engineer to see if cleanroom manufacturing and assembly is the best option for your project.






