Ronkonkoma, NY, August 11, 2021
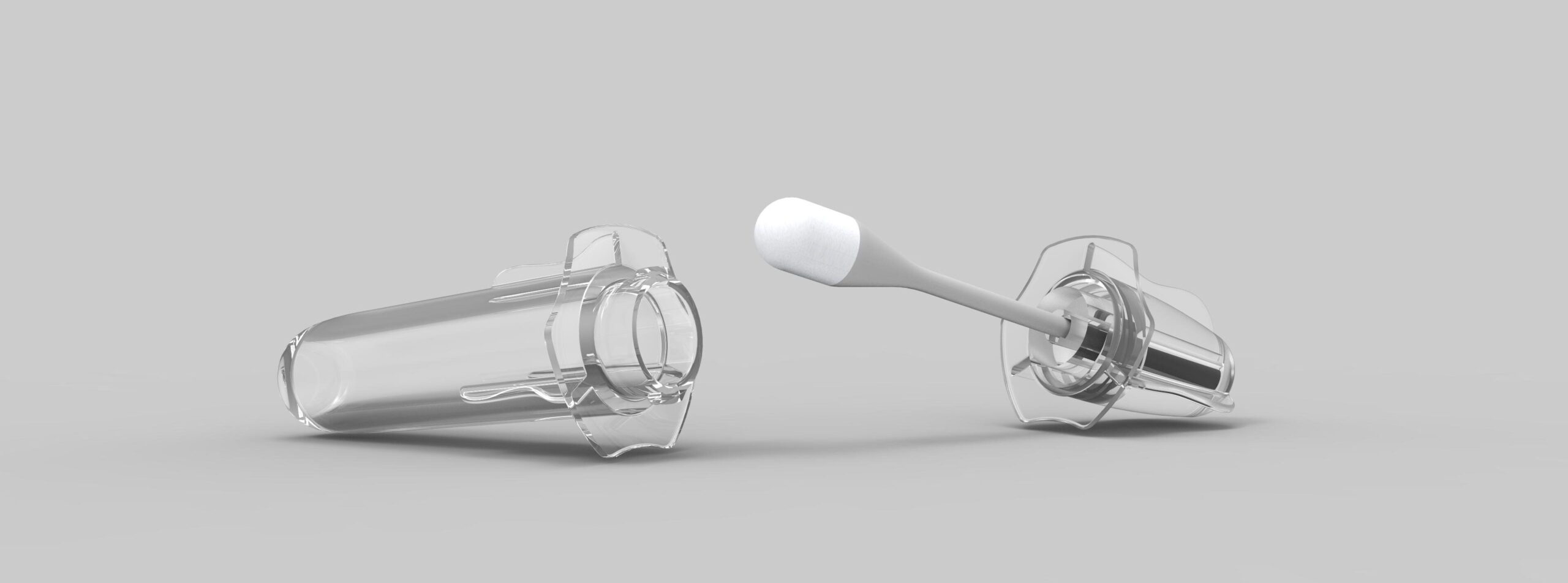
Natech Plastics has finalized the design, manufacture and assembly of its custom extraction tube. The extraction tube, used in diagnostics applications, such as COVID-19 testing, was engineered, tested and manufactured by Natech Plastics. This custom product solves supply chain issues for companies specializing in rapid diagnostics tests.
“Our customers shared their struggle in acquiring reliable extraction tubes from the current available suppliers. As a partner in their success, our team went to work to create a functional and customizable solution for the diagnostics industry. Our clients now source extraction tubes domestically from us and have confidence in the quality and performance they receive from Natech,” shares Thomas Nagler, CEO of Natech Plastics.
The extraction tube went through various iterations and rigorous testing to ensure proper quality and part functionality. Testing included material testing, permeability testing, leak testing, and functional testing. The final product has the necessary clarity, ductility, and permeability to service clients in the diagnostics market.
Natech invested in additional technology and equipment to support this product, including testing equipment, filling stations, and foil sealing machines. Clients can customize the extraction tubes to have a foil seal or plastic cap, and custom packaging for their application.
Learn more about Natech Plastics at www.natechplastics.com or view Extraction Tube Product Spotlight here: https://natechplastics.com/custom-extraction-tubes-for-antigen-tests/
About Natech Plastics
Natech Plastics is a contract development and manufacturing firm, located in Ronkonkoma, NY. Natech specializes in offering end-to-end services for clients in the medical device and diagnostics markets. Natech offers services in design, engineering, injection molding, assembly, filling and sealing, and packaging. Natech is certified ISO 9001, ISO 13485 and an FDA-registered facility, and operates an ISO class 8 cleanroom.