Controlling Product with a Quality Process
Natech Implements Quality Through Every Stage of Your Project
Natech exceeds regulatory quality expectations and requirements. Our quality team adheres to industry-standard practices, incorporates quality throughout your product’s lifetime, and customizes quality control plans that best fit your project.
Quality Systems
Natech’s certifications ensure implementation and maintenance of quality management systems in our processes. These systems ensure excellent quality for your parts, and a repeatable and traceable process.

Our ISO13485:2016 certification maintains the design, production and contract manufacture of medical devices. Our ISO 9001:2015 certification maintains the design, manufacture, assembly, and secondary operations of custom injection molded parts. Natech is also an FDA-registered facility.
Quality Process
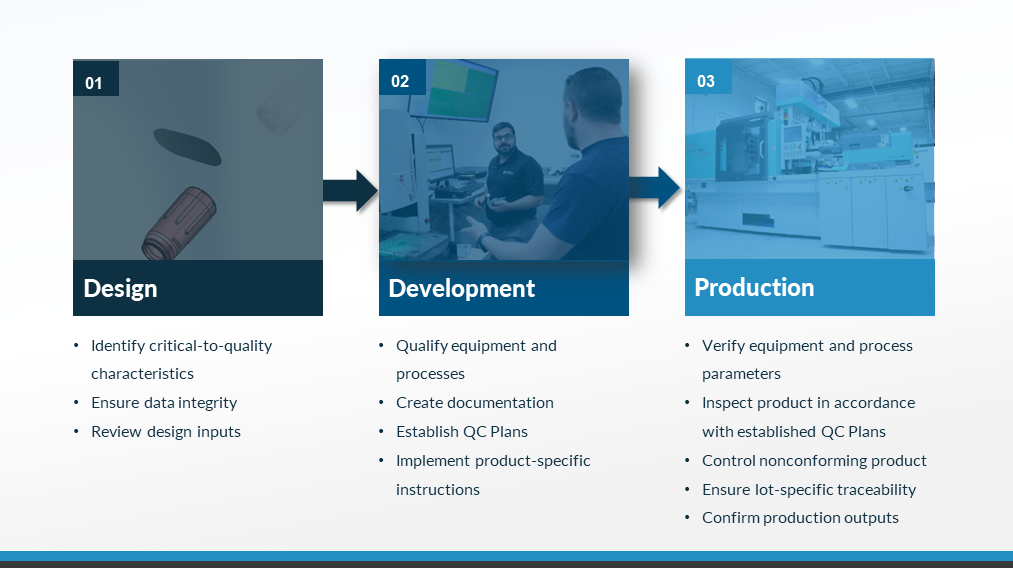
Natech’s quality process approach involves considering quality from product design all the way through final product.
Our team incorporates quality during the design for manufacturing phase. Our customers have confidence in quality of designs they receive from Natech. This phase allows our quality team to identify critical-to-quality characteristics, ensure data integrity, and review design inputs.
During product development, Natech qualifies equipment and processes, creates documentation, establishes QC plans, and implements product-specific instructions.
During production, our quality team verifies equipment and process parameters and inspects product in accordance with established QC plans. These processes control any nonconforming product, ensure lot-specific traceability, and confirm production outputs.
Customized QC Plans
Natech meets quality control requirements with customized quality control plans. Each QC plan is product-specific and tailored to the requirements of the product.
QC plans typically have a minimum of three sections, including the first in-audit inspection, in-process inspections, and final inspection. If a nonconformity is discovered during one of the inspections, our team immediately takes action to mitigate.

Natech can make custom fixturing for parts for form-fit-function tests and any quality and manufacturing protocols.
Verification and Process Control
In-process inspections are used for process control certainty.


Every product or component has a custom verification process. Inspection processes run on three shifts, with trained personnel for each process. Processes are typically checked twice per shift for visual, dimensional, and functional specifications requirements. However, Natech can create a plan with any combination of inspections that best fits your project.